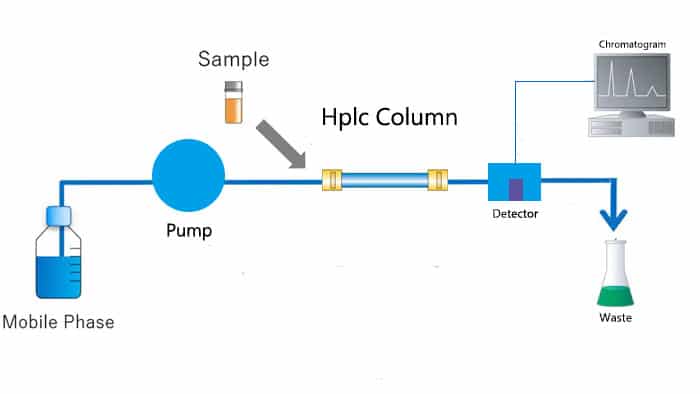
High-Performance Liquid Chromatography (HPLC), the most widely utilized branch of chromatography, serves as an analytical technique for separating, identifying, and quantifying components from mixtures. Operating via a high-pressure infusion system, individual solvents with varying polarities or proportions of mixed solvents, such as the mobile phase and buffer, are pumped into a stationary phase chromatographic column. This process effectively separates each component within the column before they are detected and tested, completing the sample analysis. HPLC has emerged as a fundamental application in separation analysis technology across diverse fields including chemistry, medicine, industry, agriculture, and commodity inspection.
The operational mechanism and principle of HPLC is detailed as follows:
Stationary Phase and Mobile Phase:
Stationary phase, as the vital component of HPLC and lies in its chromatographic column, comprised of tiny porous particles. These particles are coated or bonded with diverse chemicals on their surfaces. The selection of the stationary phase relys upon the nature of the sample to be separated. This could encompass chemically bonded phases on a silicone matrix, or non-polar, polar, or ion exchange resins.
The mobile phase, on the other hand, constitutes a blend of one or more solvents coursing through the chromatographic column under high pressure (typically ranging from 10 to 600 bar). The choice of mobile phase is critical, aiming for optimal complementarity with the stationary phase to achieve better separation efficacy. For instance, in reverse-phase HPLC, the mobile phase commonly consists of a mixture of organic solvents and water, whereas in normal-phase HPLC, a polar solvent may serve as the mobile phase.
Sample Injection and Separation:
The sample solution is precisely introduced into the continuously streaming mobile phase via a sampler. As the sample traverses the chromatographic column, its various constituents sustain differential retention due to their distinct affinities or distribution coefficients with the stationary phase chemicals.
Detection and Recording:
Successive to outflowing the chromatographic column, the components undergo detection via a series of detectors including UV, fluorescence, differential refractive index, conductivity, or mass spectrometry detectors. These detectors transform chemical signals into electrical signals, which are then captured and converted into real-time chromatograms by HPLC data processing systems.
Data Analysis:
Upon the conclusion of a run, compound identification and quantitative analysis can be conducted based on specific characteristics such as the position (retention time), area (concentration), or peak shape of each component peak observed on the chromatogram.
But we often encounter some problems when doing HPLC experiments, and we will list the common issues below:
Frequently Asked Questions
1 Why does retention time drift or change rapidly?
About drift:
- Temperature control is not reasonable; the solution is to use a constant temperature device and keep the column temperature stable;
- The mobile phase changes; the solution is to prevent evaporation and reaction of the mobile phase;
- The column is not well balanced; it needs to balance the column for a longer time.
On the question of rapid change
- The flow rate changes, the solution is to reset the flow rate so that it remains stable;
- there are bubbles in the pump that can be driven out of the bubble by exhaust operation;
- The mobile phase is not appropriate; the solution is to change the mobile phase or make the mobile phase properly mixed in the control room.
2 The cause of towing or double peaking?
- If the sieve plate is blocked or the column fails, the solution is to reverse the column, replace the sieve plate or replace the column;
- There is a disturbance peak, the solution is to use a more extended column; Change the flowing phase or replace the selective column;
- The column may be overloaded, reducing the amount of sample.
3 Main reasons and solutions for insufficient HPLC sensitivity
- Insufficient sample size, the solution is to increase the sample size;
- The sample did not flow out of the column. The flowing phase or column can be changed according to the chemical properties of the sample.
- The sample does not match the detector. Adjust the wavelength or change the sensor according to the chemical properties of the sample;
- Detector decay too much. Adjust the attenuation;
- The detector time constant is too large. The solution is to reduce the time parameters;
- Detector pool window pollution. The solution is to clean the pool window;
- There are bubbles in the test tank. The solution is exhaust;
- The recorder pressure measuring range is inappropriate. Adjust the voltage range;
- The improper flow rate of the flowing phase. Adjust the flow rate;
- The attending detector and recorder exceed the correction curve. The solution is to check the recorder and detector and recalibrate the correction curve.
4 When doing HPLC analysis, the column pressure is unstable. Why? How to solve it?
- there is air in the pump, and the solution is to clear the air in the pump, the solvent degassing treatment;
- If the proportional valve fails, replace the proportional valve;
- pump gasket damage, replace the gasket can;
- the bubble in the solvent, the solution is to the solvent degassing, when necessary to change the degassing method;
- System leak detection, find out the leak point, the seal can;
- Gradient elution, when pressure fluctuations are normal.





